Chemistry Study of Gas laws Exercise7
Please Select
Q1 What do you understand by gas?
Q2 Give the assumptions of the kinetic molecular theory
Q3 During the practical in the lab when hydrogen sulphide gas having offensive odour is prepared for some test, we can smell the gas even 50 metres away. Explain the phenomenon.
Q4 What is diffusion ? Give an example to illustrate
Q5 How is molecular motion related to temperature?
Q6 State (i) the three variables for gas laws ii) S.I units of these variables.
Q7 (a) State Boyle’s Law.
(b) Give its
(i) mathematical expression,
(ii) graphical representation and
(iii) significance.
Q8 Explain Boyle’s law on the basis of the kinetic theory of matter.
Q9 The molecular theory states that the pressure exerted by a gas in closed vessel results from the gas molecules striking’ against the walls of the vessel. How will the pressure change if :
(a) the temperature is doubled keeping the volume constant
(b) the volume is made half of its original value keeping the temperature constant?
Q10 (i)State Charles law
(ii) Give its
(i) Graphical representation,
(ii) mathematical expression and
(iii) Significance.
Q11 Explain Charles’s law on the basis of the kinetic theory of matter.
Q12 Define absolute zero and absolute scale of temperature. Write about the relationship between °C and K.
Q13 (a) What is the need for the Kelvin scale of temperature?
(b) What is the boiling point of water on the Kelvin scale? Convert it into a centigrade scale.
Q14 (a) Define S.T.P.
(b) Why is it necessary to compare gases at S.T.P.?
Q15 Write the value of
a) Standard temperature in
i) °C ii) K
b) Standard pressure in
i) atm ii) mmHg iii) cm Hg iv) torr
Q16 What is the relationship between the Celsius and the Kelvin scales of temperature?
Q17 State the laws which are represented by the following graphs.
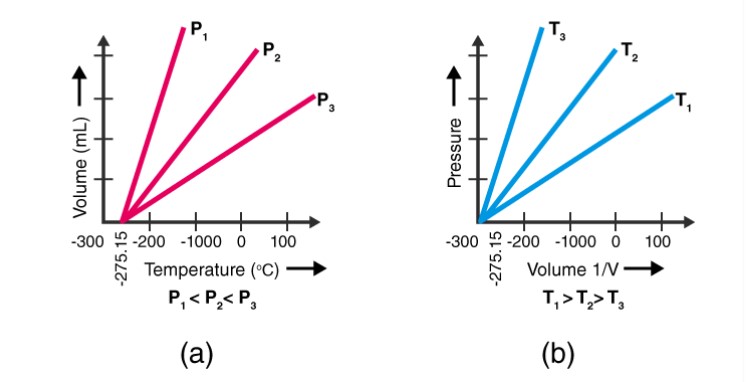
Q18 Give reasons for the following
(1) All temperatures in the absolute (Kelvin) scale are in positive figures.
(2) Gases have a lower density compared to that of solids or liquids.
(3) Gases exert pressure in all directions.
(4) It is necessary to specify the pressure and temperature of gas while stating its volume.
(5) The inflating balloon seems to violate Boyle’s law.
(6) Mountaineers carry oxygen cylinders with them.
(7) Gas fills completely the vessel in which it is kept.
Q19 How did Charles’s law lead to the concept of an absolute scale of temperature?
Q20 What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?
Q21 State the following
(a) Volume of a gas at 0 Kelvin.
(b) The absolute temperature of a gas at 7°C.
(c) Gas equation.
(d) Ice point in absolute temperature.
(e) S.T.P. conditions
Q22 Choose the correct answer (a) The graph of PV vs P for gas is
(i) parabolic
(ii) hyperbolic
(iii) a straight line parallel to X-axis
(iv) a straight line passing through the origin
(b) The absolute temperature value that corresponds t 27°C is
(i) 200K
(ii) 300K
(iii) 400K
(iv)246K
c) A volume-Temperature relationship is given by
(i) Boyle
(ii) Gay Lussac
(iii) Dalton
(iv) Charles
(d) If the pressure is doubled for a fixed mass of gas, its volume will become
(i) 4 times
(ii)1/2 times
(iii)2 times
(iv) No change
Q23 Match the following:
| Column A | Column B |
|---|---|
| a) Cm3 | i) Pressure |
| b) Kelvin | |
| c) Torr | iii) Volume |
| d) Boyle’s law | iv) =v1/t1 |
| e) Charles law | v) pv/t=p1v1/t1 |
| vi) Temperature |
Q24 Correct the following statements.
(a) The volume of a gas is inversely proportional to its pressure at a constant temperature.
(b) The volume of a fixed mass of a gas is directly proportional to its temperature, pressure remaining constant.
(c) 0°C is equal to zero Kelvin.
(d) Standard temperature is 25°C.
(e) The boiling point of water is 273 K.
Q25 Fill in the blanks
(a) The average kinetic energy of the molecules is proportional to the ………….
(b) The temperature on the Kelvin scale at which molecular motion completely ceases is called ……….
(c) If the temperature is reduced to half, …….would, also reduce to half.
(d) The melting point of ice is ……….. Kelvin.
Contact Us
