Chemistry Atomic Structure Important Questions
Q1
Name the Following:
(i) Name the scientist who discovered electrons using cathode ray experiments.
(ii) What is the name of the model that represents an atom as a positively charged nucleus
surrounded by electrons in specific energy levels?
(iii) Identify the type of bond formed when electrons are transferred from one atom to another.
(iv) Name the particle in the nucleus of an atom that carries a positive charge.
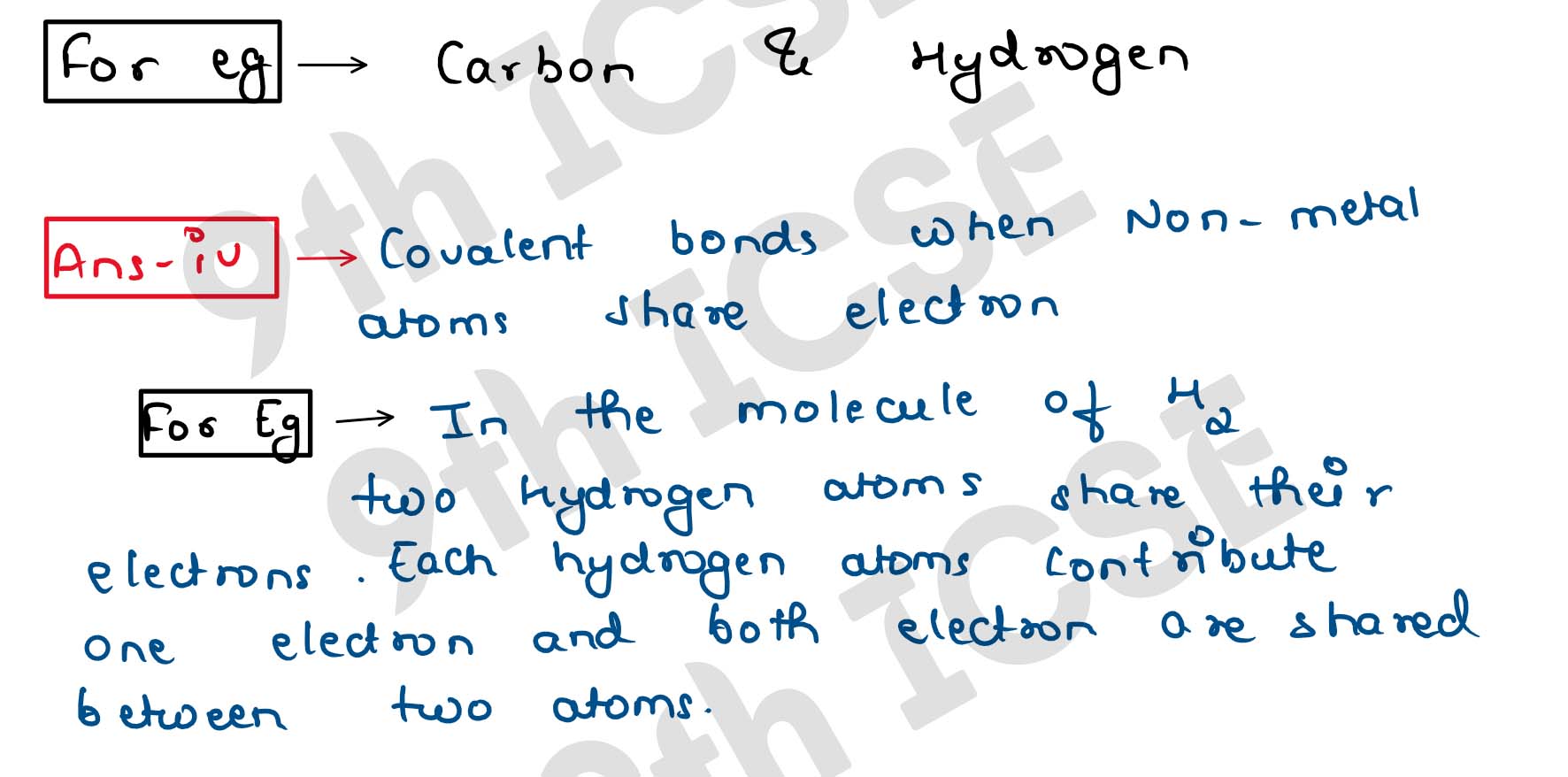
(iv) What is the term for a chemical bond formed by the sharing of electrons between two atoms?
Solution :

Q2
Fill in the Blanks:
(i) The atomic number of an element is equal to the number of ...... in its nucleus.
(ii) In Rutherford's gold foil experiment, most of the alpha particles passed through the foil because
atoms are mostly ........
(iii) An ionic bond is formed between a metal and a ........
(iv) The chemical symbol of an element represents its ........
(v) Covalent bonds are formed between ......
Solution :

Q3
(i) Describe the process of ionic bonding using an example.
(ii) Discuss the concept of isotopes and provide an example of an element that exists in isotopic
forms.
(iii) Explain the formation of a covalent bond with the help of an example. Include the concept of
shared electrons.
(iv) Describe the Rutherford's gold foil experiment and discuss its contributions to our
understanding of the atomic structure.
Solution :



Contact Us
